A Business with the Aim of Contributing to People’s Health
Providing new value by fusing advanced pharmaceutical process knowledge and human- centered automation
Founded in 1963 in Spain, for nearly 60 years Azbil Telstar has provided equipment, environmental systems, and services for pharmaceutical factories, pharmaceutical laboratories, and hospitals. The company actively incorporates the azbil Group’s advanced measurement and control technology, continuously enhancing the equipment and services it provides in its aim to provide new value that contributes to the health of people around the world.

Jochen Dick
Vice Chairman
Azbil Telstar, S.L.U.
- Offering experience and know-how to markets that contribute to people’s health
- A one-stop provider of pharmaceutical equipment and a wide range of services from process design to verification
- Continuous product and service enhancement through joint development and technological fusion
Offering experience and know-how to markets that contribute to people’s health
Life Automation (LA) is positioned as the third pillar of the azbil Group’s business, alongside Building Automation (BA) for buildings and Advanced Automation (AA) for industry. In the LA business area, Azbil Corporation is developing a life science engineering business for markets that contribute to people’s health. This business provides solutions that integrate next-generation manufacturing equipment with environmental systems, drawing inspiration from automation technology. Azbil Telstar, S.L.U., which joined the azbil Group in 2013, is responsible for this business. At the time of its founding in Spain in 1963, the company manufactured and sold vacuum pumps. It later utilized that technology in pharmaceutical production equipment, and as an equipment manufacturer has cultivated technology and know-how related to pharmaceutical manufacturing for close to six decades. From these foundations, today the company develops, manufactures, and sells lyophilizers, sterilizers, isolator equipment for pharmaceuticals, clean rooms, etc., and provides consulting, engineering, and other services related to the construction of pharmaceutical clean-room production facilities. In addition, Azbil Telstar manufactures and sells biosafety cabinets, ultra-low temperature freezers, and laboratory freeze dryers to hospitals and research facilities. It boasts bases in 11 countries in Europe, North America, Latin America, and Asia, and is expanding globally by manufacturing a wide variety of equipment at its factories in Spain, the United Kingdom, and Shanghai, China.
A one-stop provider of pharmaceutical equipment and a wide range of services from process design to verification
Azbil Telstar has four main businesses. The first covers life science equipment, mainly involving the manufacture and sale of various types of equipment used in pharmaceutical processes. A sample product is the lyophilizer. Better known as a freeze-dryer, a lyophilizer freezes liquid chemicals, lowers the sublimation point by lowering the atmospheric pressure, and vaporizes the moisture for low-temperature drying. By freeze-drying medicines and other aqueous solutions, it is possible to store them for a long time at room temperature. In addition, the company makes isolators, which are widely used to protect workers handling substances harmful to humans in pharmaceutical processes. The company also manufactures sterilizers, which are used for sterilizing equipment used to produce devices like syringes for COVID-19 vaccines, or sterilizing the final product in order to make it stable and safe.
The second main business of the company is clean room solutions. In the manufacture of pharmaceuticals, it is necessary to maintain a clean environment in which the number of fine airborne particles and microorganisms is kept below a certain level. Because Azbil Telstar has a high level of expertise in manufacturing processes for pharmaceuticals, and is itself an equipment supplier, it designs the manufacturing facilities needed to realize such an environment, and offers support in areas from equipment procurement to construction management. Its strengths include not only designing equipment, but also the ability to create systems that prevent data falsification, by means such as equipment validation—checking whether or not the production process on that equipment is operating properly—along with comprehensive consultation services.
The third business area is the consulting and customer service business, which undertakes annual external audit work for manufacturers in countries such as India and China where pharmaceutical companies procure raw materials. Azbil Telstar checks whether good manufacturing practices (GMPs) are used and whether the product quality of raw material manufacturers complies with local laws and regulations, and submits a report to the requesting pharmaceutical company. Also, it provides regular inspections of pharmaceutical production processes and calibration*1 for equipment that measures physical quantities like temperature and pressure.
In its fourth business, in the research and medical areas, the company manufactures and provides lyophilizers for use in experiments, bio-safety cabinets, ultra-low temperature freezers, etc., for pharmaceutical and biological laboratories and medical sites in hospitals. This equipment is useful for the handling and safe storage of samples collected from patients, such as PCR tests for the COVID-19 coronavirus.
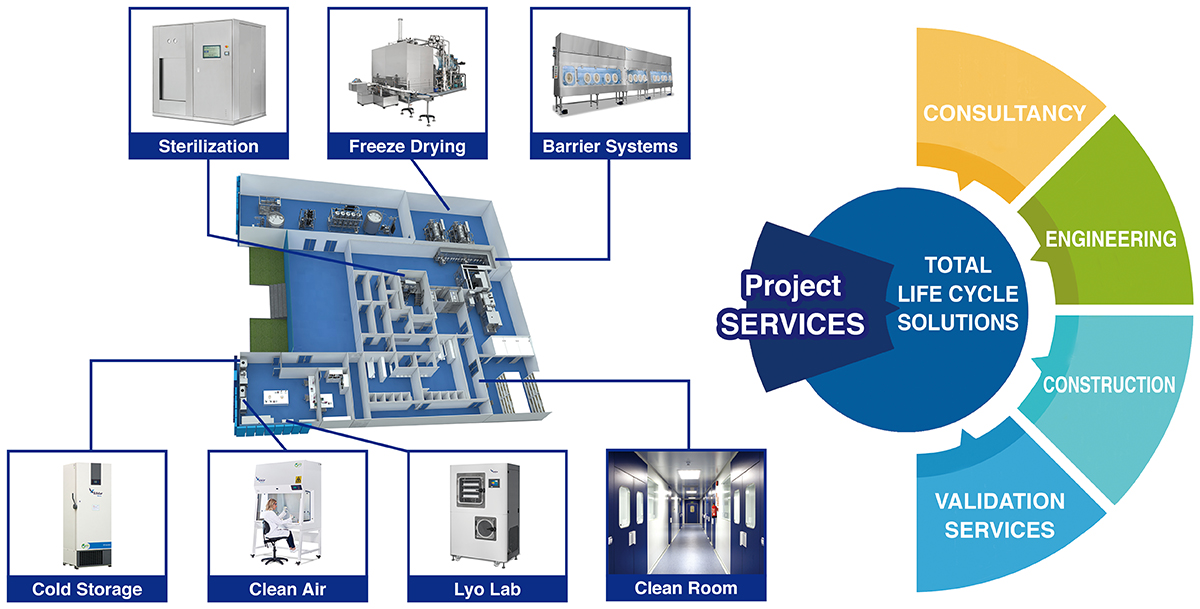
Major products and businesses of Azbil Telstar
Continuous product and service enhancement through joint development and technological fusion
In addition, Azbil Telstar is constantly working to improve the efficiency of its products, reduce costs, and improve quality by utilizing Azbil’s measurement and control technology. Already, in cooperation with Azbil’s technology headquater departments, a new automatic loader/unloader has been developed that loads vials of pharmaceuticals into a lyophilizer and unloads them after the freeze-drying process. This technology reduces workers’ risk of inhaling substances when removing products from the lyophilizer while also preventing dust and bacteria from entering the substances inside the equipment, helping to ensure cleanliness, detergency, and sterility in the freeze-drying process.
Azbil Telstar is also working to improve the quality of its products by incorporating azbil flowmeters into its isolators. Isolators, used mainly in the manufacture and development of pharmaceuticals, completely separate their interior from the surroundings, providing an aseptic environment in an isolated unit for pharmaceutical processes, or protecting operators who handle harmful or toxic substances. Decontamination within the isolated unit is essential for maintaining a sterile environment inside. Azbil Telstar’s decontamination equipment mixes hydrogen peroxide solution evenly with air and sprays it. Accurate measurement of the very small amount of spray is difficult to do. To overcome this challenge and improve the quality of decontamination, Azbil Telstar incorporated Azbil’s micro flow rate liquid flowmeter into the decontamination equipment to measure the actual flow rate of hydrogen peroxide in the flow path to the spray port. All isolators provided by the company now have built-in Azbil-made micro flow rate liquid flowmeters.
While leading the life sciences engineering portion of the LA business and focusing on SDG-related initiatives, Azbil Telstar continues to strengthen its products and services by actively incorporating the advanced knowledge, know-how, and technology related to measurement and control that the azbil Group has cultivated over many years. It is the company’s earnest desire to provide value that contributes to the health of people around the world through its business activities.
The interview for this article was conducted in May 2022. At that time Jochen Dick was the CEO of Azbil Telstar.
*1 Calibration
The task of investigating how much error there is between the measured value and the true value under the specified conditions. Calibration can include adjustment of the measuring instrument.
This article was published on October 1, 2023.






